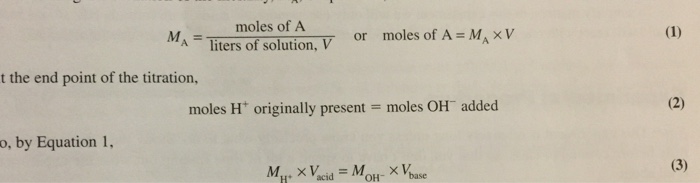Advance study assignment molar mass of an acid – In the realm of chemistry, the molar mass of an acid holds immense significance, providing a fundamental understanding of acid properties and their behavior in reactions. This advance study assignment delves into the concept of molar mass, its calculation, and its applications in acid-base chemistry.
By exploring the intricacies of molar mass, students will gain a deeper appreciation for the quantitative aspects of acid chemistry, enabling them to accurately predict and analyze the outcomes of acid-base reactions.
Molar Mass of an Acid: Advance Study Assignment Molar Mass Of An Acid
The molar mass of an acid is a measure of its mass per mole. It is a fundamental property of an acid and is used in various chemical calculations. The molar mass of an acid can be calculated using the following formula:
Molar mass = (Atomic mass of each element in the acid) x (Number of atoms of each element in the acid)
The units of molar mass are grams per mole (g/mol).
Methods for Determining Molar Mass
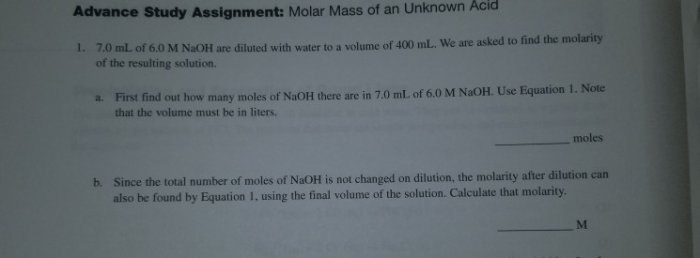
The molar mass of an acid can be determined using various methods, including titration. In titration, a known volume of a base is added to a known mass of the acid until the acid is neutralized. The molar mass of the acid can then be calculated using the following formula:
Molar mass = (Mass of acid) x (Molarity of base) x (Volume of base) / (Volume of acid)
where:
- Mass of acid is in grams
- Molarity of base is in moles per liter
- Volume of base is in liters
- Volume of acid is in liters
Applications of Molar Mass in Acid-Base Reactions, Advance study assignment molar mass of an acid
The molar mass of an acid is used in stoichiometric calculations involving acids. For example, it can be used to determine the amount of acid required to neutralize a given amount of base. The molar mass of an acid is also used in acid-base titrations to determine the concentration of an unknown acid.
Advance Study of Molar Mass
In advanced chemistry, the concept of molar mass is extended to include polyprotic acids. Polyprotic acids are acids that can donate more than one proton. The molar mass of a polyprotic acid is the sum of the molar masses of all the hydrogen atoms that can be donated.
The molar mass of an acid can also be used to predict its properties. For example, acids with a higher molar mass tend to be stronger acids.
FAQ Resource
What is the formula for calculating molar mass?
Molar mass = (Mass of molecule in grams) / (Number of moles of molecule)
How is molar mass used in acid-base titrations?
Molar mass is used to determine the concentration of an unknown acid by reacting it with a known volume of a base of known concentration.
What is the relationship between molar mass and acid strength?
In general, acids with lower molar masses tend to be stronger acids.