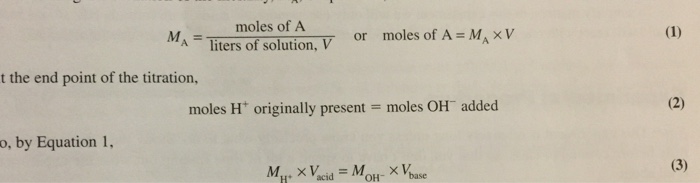How many moles are in 68 grams of copper hydroxide? This seemingly straightforward question invites us on a captivating journey into the realm of chemistry, where we will unravel the intricacies of mole calculations, explore the applications of copper hydroxide, and uncover the significance of understanding the relationship between mass and moles.
Copper hydroxide, a compound with the chemical formula Cu(OH)2, plays a crucial role in various industrial processes. Determining the number of moles present in a given mass of copper hydroxide is essential for optimizing its applications and ensuring precise experimentation.
This exploration will equip you with the knowledge and skills to confidently navigate the fascinating world of mole calculations and unravel the mysteries of copper hydroxide.
How Many Moles are in 68 Grams of Copper Hydroxide?

Copper hydroxide is an inorganic compound with the chemical formula Cu(OH) 2. It is a pale blue or green solid that is insoluble in water. Copper hydroxide is used in a variety of applications, including as a fungicide, a mordant in dyeing, and a catalyst in organic reactions.
Overview
A mole is a unit of measurement used to express the amount of a substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
The relationship between moles and mass is given by the following equation:
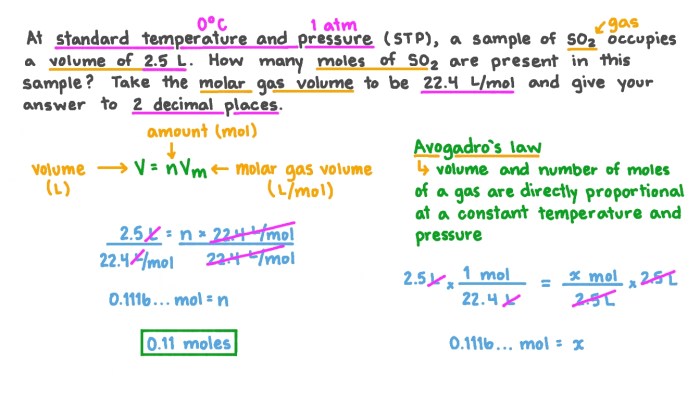
moles = mass (grams) / molar mass (grams per mole)
The molar mass of copper hydroxide is 97.56 grams per mole.
Calculations, How many moles are in 68 grams of copper hydroxide
To convert 68 grams of copper hydroxide to moles, we use the following formula:
moles = mass (grams) / molar mass (grams per mole)
Substituting the given values into the equation, we get:
moles = 68 grams / 97.56 grams per mole
Therefore, 68 grams of copper hydroxide is equal to 0.697 moles.
Chemical Equation
The balanced chemical equation for copper hydroxide is:
Cu(OH)2→ CuO + H 2O
In this equation, one mole of copper hydroxide produces one mole of copper oxide and one mole of water.
Therefore, the number of moles of copper hydroxide in the equation is equal to the number of moles calculated in step 2, which is 0.697 moles.
Applications
Copper hydroxide is used in a variety of applications, including:
- As a fungicide
- As a mordant in dyeing
- As a catalyst in organic reactions
- As a pigment in paints and ceramics
- As a water treatment chemical
The number of moles of copper hydroxide needed for a specific application will depend on the desired outcome.
Commonly Asked Questions: How Many Moles Are In 68 Grams Of Copper Hydroxide
What is the molar mass of copper hydroxide?
The molar mass of copper hydroxide (Cu(OH)2) is approximately 97.56 grams per mole.
How many moles of copper hydroxide are present in 100 grams of the compound?
To calculate the number of moles, divide the mass (100 grams) by the molar mass (97.56 grams per mole). Therefore, 100 grams of copper hydroxide contain approximately 1.025 moles.
What are the common applications of copper hydroxide?
Copper hydroxide finds applications in various industries, including water treatment, fungicides, and the production of other copper compounds.